The most comen causes of chronic kidney disease CKD are diabetes and hypertension. diabetes is the most cause of CKD with 45% of diabetics will develop it during their lives. more than 27% of hypertensive patients will develop CKD. eating diabetes and kidney disease low-carb diet is crucial to prevent side effects of high blood sugar as well as healing insulin resistance and diabetes. But what is diabetes and kidney disease diet that you must consider to manage CKD and diabetes simultaneously for the best outcome? (I)
today we will discuss diabetes and kidney disease diet proven by practice and literature and its ability to manage blood sugar, enhance kidney function, reduce creatinine, and uric acid, and increase power, stamina, and Glomarius filtration rate.
Can you have diabetes and kidney disease at the same time?
of course, 50% of diabetics have kidney disease. especially if you do not control blood sugar reading near the standard values. having Type 1 diabetes has been estimated to increase the risk of developing CKD during a lifetime to more than 70%.(II)
this high prevalence of CKD among type 1 diabetes is caused by the sustained elevation of HBA1c above 7.9 and blood sugar of 180mg/dL which represents the kidney glucose reabsorption threshold. as it appears in the Diatribe.org the Average HBA1c overall has risen up from 7.8% in 2010-2012 to 8.4% in 2016-2018. (III)
How does diabetes cause nephropathy pathophysiology?
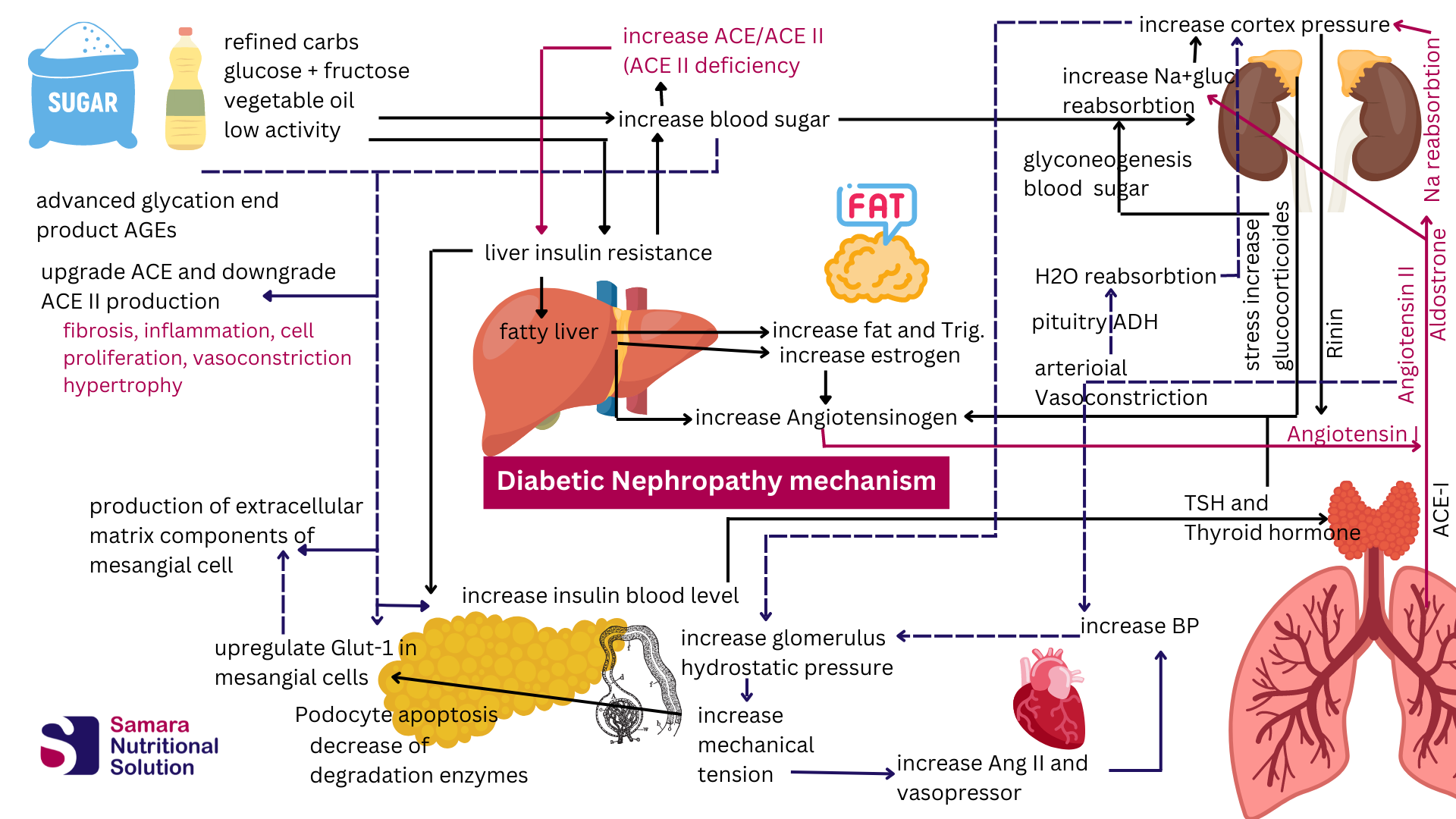
Firstly increase in glucose, refined carbohydrate, fructose. and vegetable oils with a high level of omega-6 will cause an increase in insulin resistance. As a result insulin resistance will increase beta-cells insulin secretion. secondly high level of insulin, as well as liver insulin resistance. increase lipogenesis that causes fatty liver.
progression of the fatty liver increases estrogen levels. while high levels of insulin and glucose cause an increase secretion of TSH and thyroxine. all the fatty liver, high level of estrogen, and thyroxine stimulate the activation of the angiotensinogen renin system which begin with the angiotensinogen in liver cells.
increase glucose levels and angiotensin II (Ang II) will cause a rise in blood pressure by increasing the reabsorption of sodium. high glucose causes an increase in glucose sodium pump action in the Proximal tubule. as well as sodium reabsorption in the Distal tubule as a response to Aldosterone. both sodium and glucose drag water into the adrenal cortex which increases renal cortex pressure. this causes resistance to blood flow to the glomerulus rise in glomerulus hydrostatic pressure.
angiotensin II increases arteriolar vasoconstriction which reduces blood flow to the glomerulus and leads to an increase in blood pressure. that increase is to overcome vasoconstriction as well as glomerulus hydrostatic pressure. this increase in blood pressure increases mechanical tension in the glomerulus.(IV)
Glomeruli mechanical stretch consequences
Mesangial cell
- glomerular capillary hypertension cause changes that induce glomerulosclerosis in mesangial cells and podocytes.
- alter Mesangial cell morphology from normal stellate to a straplike appearance. that reorders its axis perpendicular to the direction of stress.
- Mechanical stretch upregulates and increases glucose transport (GLUT-1) by 80% in mesangial through a TGF-β1–dependent mechanism.
- extracellular matrix components increased to support the glomeruli to resist the tension stress. this increase in size and density is amplified with a high glucose medium.
- reduce the activity of degradative enzymes that dissolve extracellular matrix components.
- mechanical stretch induces extracellular matrix deposition by interacting with specific metabolic, hemodynamic, and inflammatory pathways.
- Mechanical stretch upregulates glucose transport through a TGF-β1–dependent mechanism.
- mechanical stretch stimuli the production of renin and increase the activity of the angiotensin renin system which increases system blood pressure. (IV)
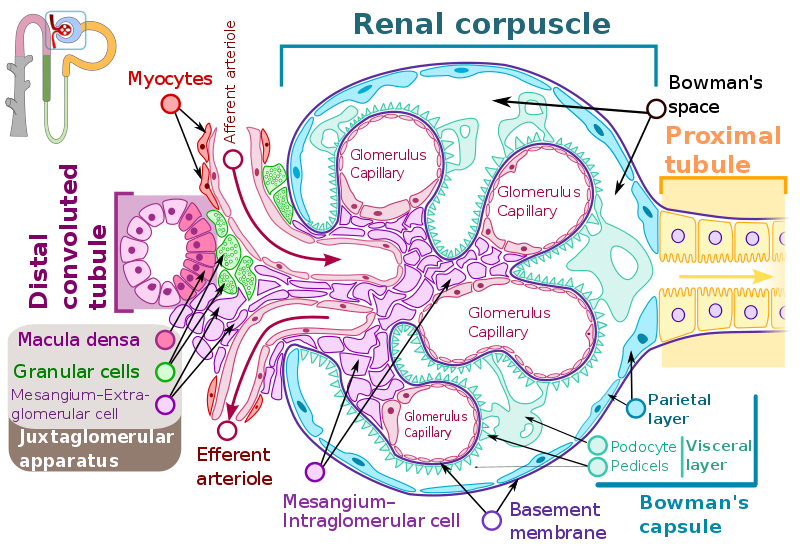
Podocyte cell
- decrease in the number and/or density of podocytes
- reduce Podocyte cell proliferation, and reduce cell adhesion to the basement membrane
- increase of angiotensin II induces apoptosis and downregulates nephrin which is related to diabetic nephropathy.(Nephrin is a protein that localizes the slit pore diaphragm of the podocyte cells)
- increase ACE/ACE2 ratio. which increases inflammation, hypertension, vasoconstriction, cell proliferation, and fibrosis
- increase blood glucose, and causes the generation of AGEs. which induces autocrine production of Ang II which cause TGF-B, fibronectin synthesis, and cell hypertrophy.(IV)
Evaluation of Chronic kidney disease
Diabetic nephropathy progress causing glomerulosclerosis caused by excess accumulation of extracellular matrix overproduced by mesangial cells. with the increased formation of advanced glycation end products.
reabsorption of glucose and sodium causes renal tissue to suffer from nephropathy with edema. that’s why Type 1 diabetic patients often suffer from enlarged kidneys with an elevation in the glomerular filtration rate.(V)
80% of Type 1 diabetic patients will progress to microalbuminuria within 15 years and 75% of their shift to end-stage renal disease within 20 years. (V)
All this stress will cause deterioration of the glomerular filtration rate (GFR) and an increase in creatinine. with the decrease in GFR patient will progress throw stages from the first until the fifth stage which needs to be put the patient on dialysis.
what is the diet to follow for a kidney?
it is clear that high glucose levels as well as glomerulus high hydrostatic pressure. causes deterioration of all kidney structures. so it is crucial to cut-off carbohydrates to manage and normalize blood sugar. while controlling blood pressure within the normal range.
yes, by cutting carbohydrates you will eat diabetes and kidney disease diet such as a modified ketogenic diet. but of course, you need a well-formulated kidney-friendly keto diet that does not put more pressure on the compromised kidneys.
Do not forget that the kidney will begin to join the liver gluconeogenesis. and produce 40% glucose synthesis in the body when the kidney gets into starvation mode.
Does Keto really benefit the kidney?
ketogenic diet is benitfical to kidney if it is done properly. to benefit the kidney in the diabetes and kidney disease diet modified keto diet. the diet should be easy on glomerulus to filter the blood
- ACE activity is reduced during starvation which reduces blood pressure by enhance vasodilation.
- reduce of insulin and glucose ingestion reduces thyroxine which causes a reduction of angiotensinogen liver production.
- Blood pressure reduction during fasting may result from low T3 levels.
- liver health improves and fat accumulated in it is burned for fuel. which reduces blood pressure and water retention.
- The kidney is powerful to produce glucose by gluconeogenesis and can synthesize glucose from amino acids. In addition at the same time kidney thrive on ketones which is an anti-inflammatory fuel source.
- quantity of ketone bodies increases 20 times more than before the keto diet.
- loss of sodium from the kidney reduces renal cortex pressure. as well as glomerulus hydrostatic pressure.
- ketogenesis can be enhanced by the sodium-glucose cotransporter 2 (SGLT2) inhibitors. By promoting urine glucose excretion through inhibition of renal glucose reabsorption. so an energy-deficient metabolic state that stimulates the endogenous production of ketones. as a compensatory energy source.
moreover ketone bodies it self have a lot of benefits:
- After a decrease in absolute podocyte number. podocytes can be partially replaced as appeared in experimental mouse models.(VI)
- ketosis with the reduction of glucose level and glucose reabsorption rate reduces renal O2 consumption which lowers free radicals and increases O2 availability to kidney tissue repair.
- glomerulus repair is possible as the podocyte compartment is more dynamic than previously believed. Bidirectional exchange with neighboring cellular compartments provides a mechanism for podocyte replacement.
- beta-hydroxybutyrate increases the protection of the kidney against different toxins. such as Paraquat which is a toxic chemical that is widely used as an herbicide
- beta-hydroxybutyrate increases tolerance to kidney ischemia and reperfusion injury. as well as prevents tissue damage and renal function decline.
- beta-hydroxybutyrate inhibits a type of programmed cell death. with a significant inflammatory component called pyroptosis.(VII)
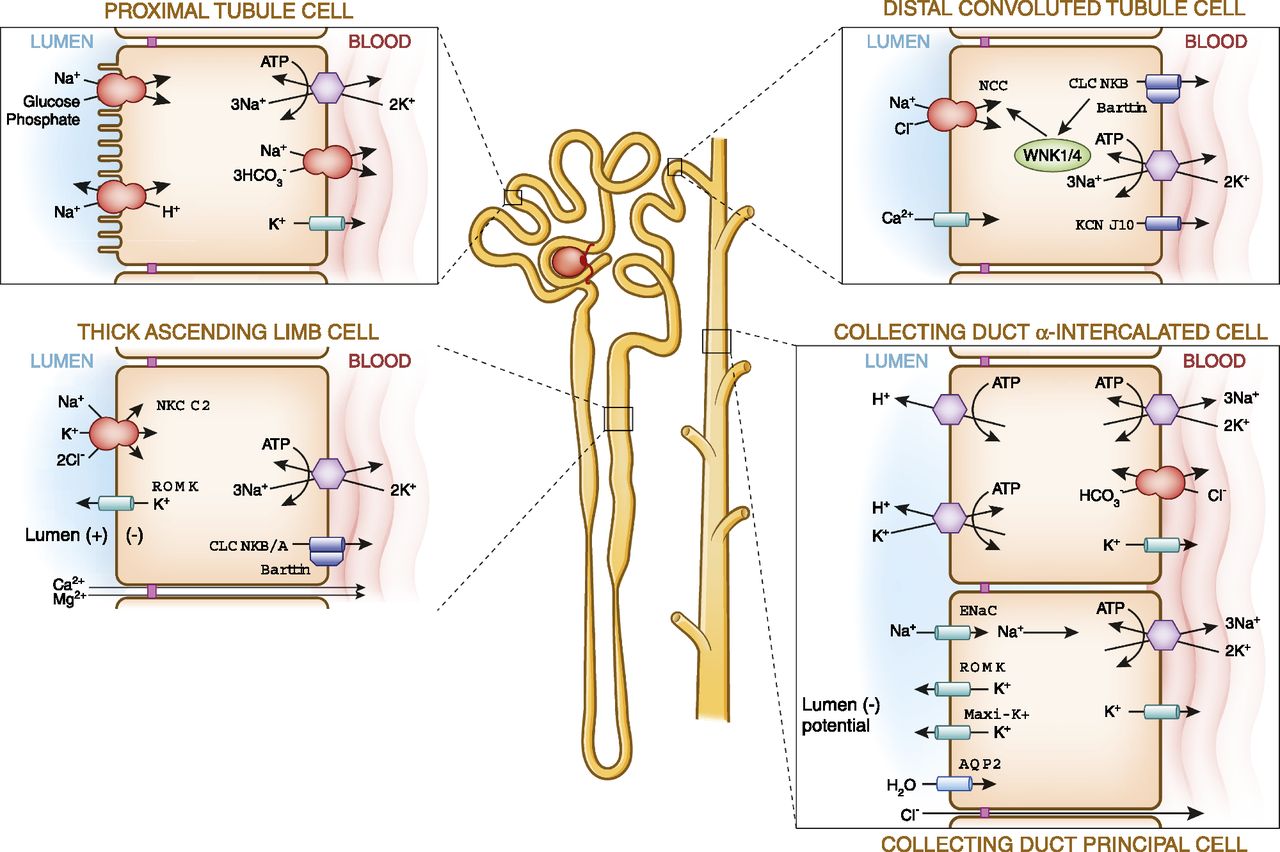
What should a diabetes and kidney disease diet?
Diabetes and kidney disease diet should reduce energy demand in kidney. as will as reduce hypertension in renal cortex. A diet low in sugar the mimic starvation will be effective in reducing ACE production. While low protein ,low phosphorus, low potassium diet will reduce energy consumed in filtration and reabsorption.
To reduce uric acid low purine diet. as will as no fructose diet. and no nuts will be effective to support kidney healing.
Carbohydrate
Carbohydrate in diabetes and kidney disease diet should be restricted. not to exceed 50gram total carb daily about 5% of daily calories. This restriction is to control blood sugar and reduce it below 80mg/dl. which will reduce reabsorbed water and salt with glucose reabsorption.
Blood sugar must be controlled by kidney friendly medication. such as SGLT-2 inhibitors for type 2 diabetics or insulin in type 1 diabetics. When using SGLT-2 inhibitors ketone levels should be measured as it cause the body to shift into ketosis.
Lipids in diabetes and kidney disease diet
All manufactured oils such as vegetable oils or margarine is restricted as it increase inflammation and induce liver insulin resistance. Fats will be the main energy source sufficient to bring total calories as requirement to sustain desired body weight.
Animal fat, Gee, virgin olive oil, and unprocessed coconut oil is best sources of fat. Percent of fat in the diet calories is 80-85%
Protein diabetes and kidney disease diet
The production of urea is closely related to the amount of protein eaten; therefore, urea can be used to estimate whether a patient with CKD is receiving the required amounts of protein. In addition, urea production serves as an estimate of the accumulation of putative uremic toxins and, thus, as a guideline for management of the diets of patients with CKD.(VIII)
Normal individuals who eat small amounts of protein have low GFR values (7). On the contrary, eating a large meal of protein transiently raises GFR
As the red blood cells ascend in the ascending vasa recta, they need to lose urea. In the absence of UT-B1, the red blood cells are unable to lose urea quickly enough and take some of the urea out of the medulla and into the bloodstream, thereby reducing the efficiency of countercurrent exchange and urine concentrating ability(II)
Only approximately 50% of the ammonia produced is excreted in urine under basal conditions. The remaining ammonia enters the systemic circulation through the renal veins. Ammonia that enters the systemic circulation undergoes almost complete metabolism in the liver; the major metabolic pathway uses HCO3− as a substrate, and generates urea. Consequently, ammonia produced in the kidney, transported to the systemic circulation, and metabolized in the liver to urea has no net acid-base benefit.
low-protein diet for at least 2 weeks have a decrease in the fractional excretion of urea (37). This results, at least in part, from the functional expression of vasopressin-stimulated urea permeability in the initial
A low protein diet (LPD) of 0.6–0.8 g/kg/day is often recommended for the management of CKD(ii). But be aware to take the ideal body weight in the case of CKD. I prefer not to exceed 0.6-0.7 g/kg.day = 45-50g/protein.day

Phosphorus
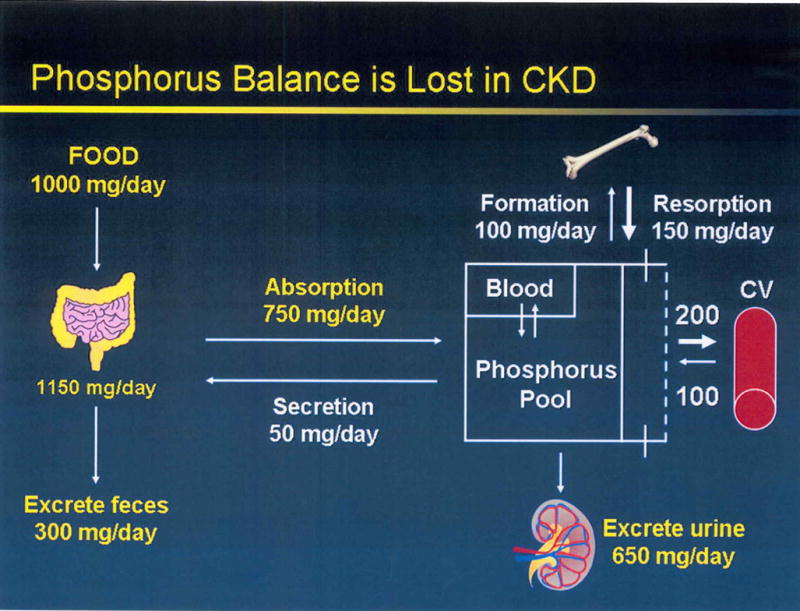
Hyperphosphatemia is an advanced state of CKD that is avoided at the first stages by increased renal extraction and a decrease in renal reabsorption. with the progression of the disease and reduction of GFR between 20 and 30ml/min/1.73m2 hyperphosphatemia become evident.
Approximately 7 g of phosphorus is filtered daily by the kidney, at physiologic levels of serum phosphorus, of which 80%-90% is reabsorbed by the renal tubules and the remainder is excreted in the urine (approximately 700 mg) equal to intestinal absorption.(VIII)
this lack of phosphate extraction power reduces extraction from 800mg/day to 650mg/day with a 20% drop. almost 10-20% of the phosphate extracted is reabsorbed in the proximal tubule. the intestine absorbs about 75%-85% of the phosphate ingested.
So if the patient ingests 800mg of phosphate as recommended for end stage kidney disease. about 650mg of phosphate is absorbed in the intestine which matches the renal extraction capacity of phosphorus.
the dietary phosphorus restriction of 800-1000mg/day or 17mg/kg of ideal body weight or standered body weight per day is recommended to CKD patients from the beginning of stage 3. in our practice performing with will formulated ketogenic diet on stage-2 patients alone without the phosphate restriction can reduce creatinine. but doing keto kidney diet (KKD) provide faster results by reduce filtration effort on glomerulus. (IX)
60g pita bread has about 120mg of phosphorus which is 20% of the daily allowance for patients with CKD. One pita bread have phosphorus equivalent 2ounces of meat from 7onces needed to reach 50g protein.
Potassium
Only 1-2% of potassium is available in the blood circulation. Potassium blood concentration should be regulated continually by the kidney. the improperly functioning kidneys lose the ability to filter fluids and electrolytes in the body, which can lead to dangerously high levels of potassium in the blood.
Reducing the amount of potassium ingested reduce the energy needed to secrete K+ to the lumen which spare ATP to get rid of creatinine and reduce kidney effort.
Reducing potassium to not more than 2000mg/day is suitable to reduce pressure on nephrons as will as reduce the risk of hyperkalemia
Can you stop diabetic kidney disease from progressing?
According to our practice by using KKD it is possible not stop progression but also to reverse nephropathy and increase GFR to patients with CKD in all stages. From stage 1 throw 5.
Our protocol aims to support relief the kidneys by reducing ATP energy demand as will as reducing glomerulus mechanical tension with the reduction of cortex static pressure.
All of our patient where put in a low potassium, low phosphorus, low protein, low purine, very low carb ketogenic very high fat diet.
Can you reverse kidney damage from diabetes?
Yes you can if the diabetes is managed properly and the kidney cortex is reduced that allow the Podocytes to regenerate and the interstitial matrix around mesangial cells to be degraded.
Yes you can if the ATP renal production capacity exceed it demand during healing time.
Podocytes are critical component of the nephron filtration barrier that has the ability to regenerate in optimum conditions.
foods to avoid with kidney disease and diabetes
- cheese and milk
- egg yolk
- processed oils
- juices
- leafy greens
- fruits
- almonds and nuts
- grains and bread
Case Studies with kidney disease and diabetes KKD diet
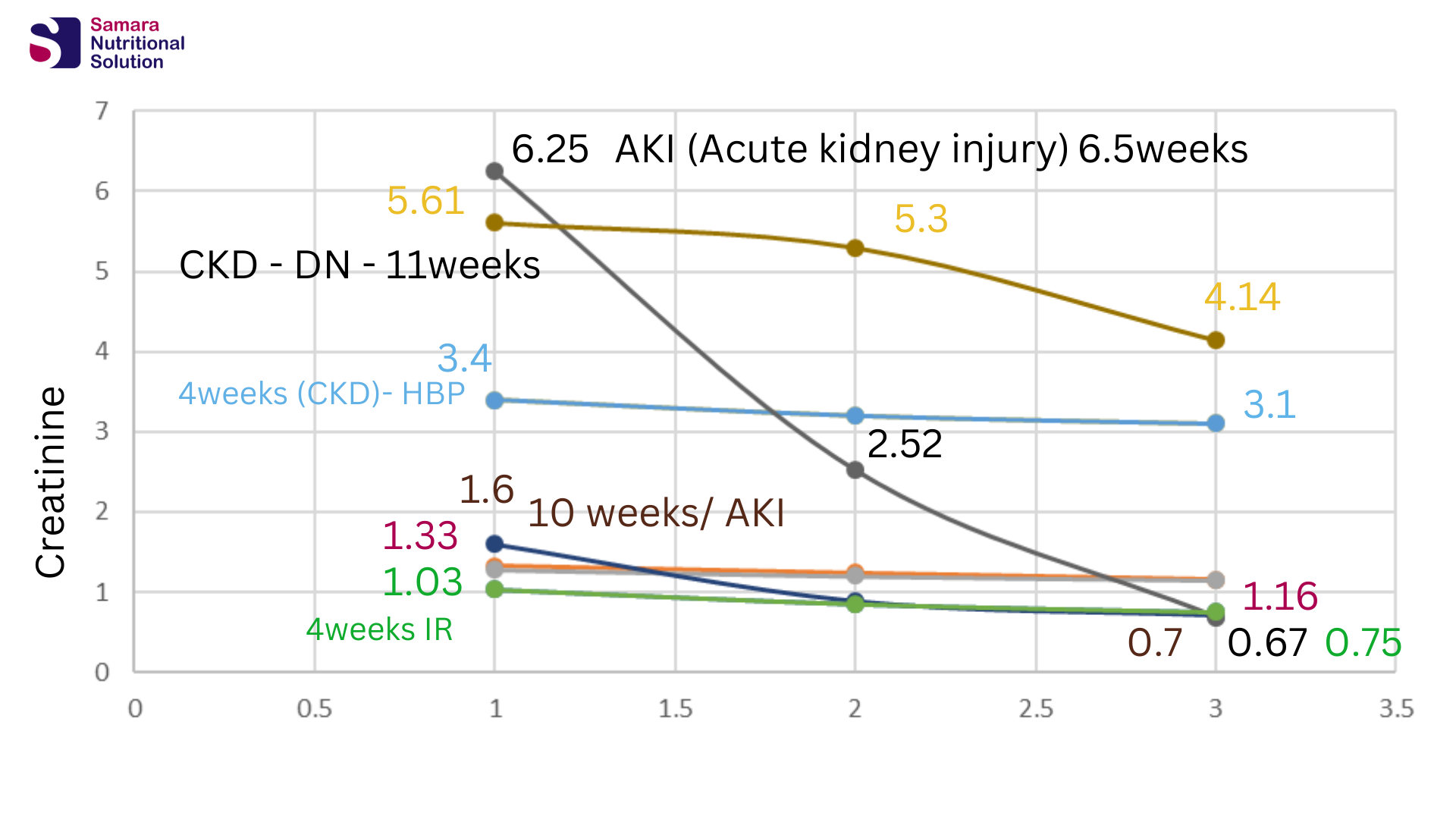
Our study on patient in our clinic show an amazing improvement in CKD and AKI.
Patient with Acute kidney injury drop values from thire high creatinine levels to normal range. Fatima from Kuwait drop here creatinine results from 6.25 to 0.69. while Yousef from Jordan dropped his values from 1.6 to 0.7 within less than 10weeks after the beginning of the program.
Basem from Jordan patient of Diabetic Nephropathy reduces his insulin units drom 30 units to 10 units only. While he is improved from stage 5 to stage 4 with an imprisve creatinine drop from 5.61 to 4.14.
Fadia from Jordan slightly improve her results even though she has a poor blood pressure control. As here blood creatinine is reduced from 3.4 to 3.1
How to contact our clinic
Our online clinic services patients all around the world by a team of highly trained registered dietitians. how are superior in the dealing with complicated metabolic health situations by using low carbohydrate and ketogenic diets.
Book your appointment on line and register to our supervision packages from our website: www.samaraketolife.com Or contact us by the clinic WhatsApp 0096275581329




refrances
II- Risk Factors for Kidney Disease in Type 1 Diabetes
III- Few Type 1s Meet A1C Goals Despite Treatment Innovations
IV– Mechanisms of Diabetic Nephropathy
V-Altered Dynamics in the Renal Lymphatic Circulation of Type 1 and Type 2 Diabetic Mice
VI- Can podocytes be regenerated in adults
VII-Ketone bodies for kidney injury and disease
VIII- Renal phosphate handling: Physiology
IX- Phosphorus metabolism in chronic kidney disease
Diet Tips for People with Diabetes and Kidney Diseas
Effect of low-carbohydrate diets high in either fat or protein on thyroid function. plasma insulin, glucose, and triglycerides in healthy young adults
Angiotensin-converting enzyme activity decreases during fasting
Antihypertensive and Renal Mechanisms of SGLT2 (Sodium-Glucose Linked Transporter 2) Inhibitors
Monitoring of Paraquat in soya products intended for animal feed
Beyond a Passive Conduit: Implications of Lymphatic Biology for Kidney Diseases
Renal Lymphatics: Anatomy, Physiology, and Clinical Implications
Effect of elevated interstitial pressure on the renal cortical hemodynamics
The ACE2/Ang-(1–7)/Mas axis can inhibit hepatic insulin resistance.
ACE/ACE2 Ratio: A Key Also in 2019 Coronavirus Disease (Covid-19)?